Research Areas
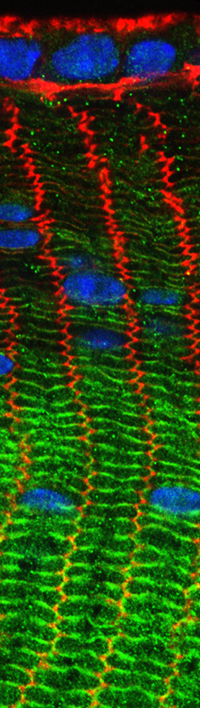
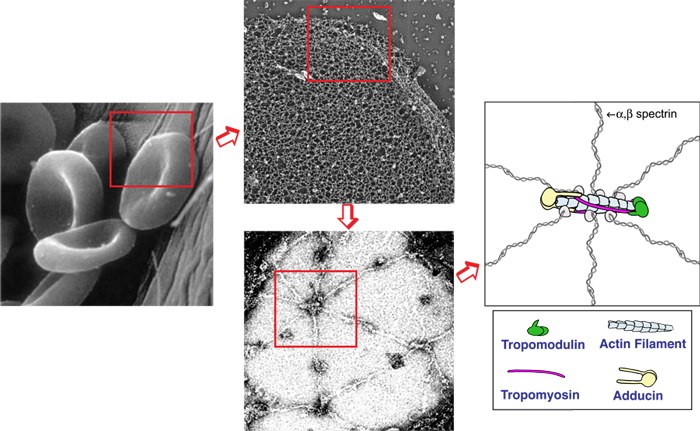
The biconcave disk shape and deformability of mammalian red blood cells (RBCs) rely upon the membrane skeleton, a viscoelastic network of short actin filaments interconnected by long spectrin tetramers in a periodic lattice. Unlike many other cell types, RBCs can be easily isolated in large quantities and contain no transcellular or cytoplasmic cytoskeleton, allowing the membrane skeleton to be studied in isolation from other populations of actin or myosin. Since periodic spectrin-actin networks are present in other cells, such as neurons and epithelial cells, the RBC membrane provides a unique paradigm for exploration of fundamental principles in membrane biology. Recently, our lab discovered that non-muscle myosin IIA (NMIIA) motors interact with the spectrin-actin network to maintain RBC biconcave shape and deformability. We are studying nanoscale lattice structure and NMIIA assembly using super-resolution fluorescence microscopy; membrane curvature and cell shape using 3D confocal microscopy; cell deformability using biomechanical assays; and physiology using hematological assays. By examining RBCs from human patients and transgenic mice with mutations in NMIIA or membrane skeleton components, we can reveal the molecular and structural basis of RBC shape, deformability, and physiology. We also collaborate with computational cell biologists to model how NMIIA forces exerted on the nanoscale network structure determine microscale membrane curvature and biconcave shapes.
Read more about the spectrin-actin lattice here.
Read more about myosin and RBC shape.
Read an article in “The Scientist” about Actomyosin and RBC shape.
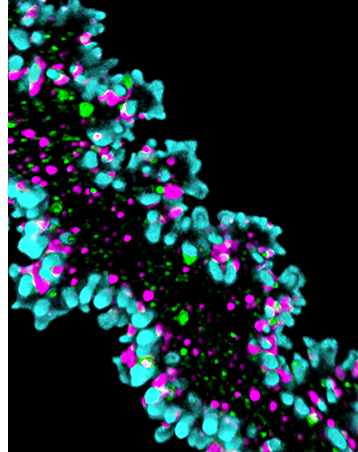
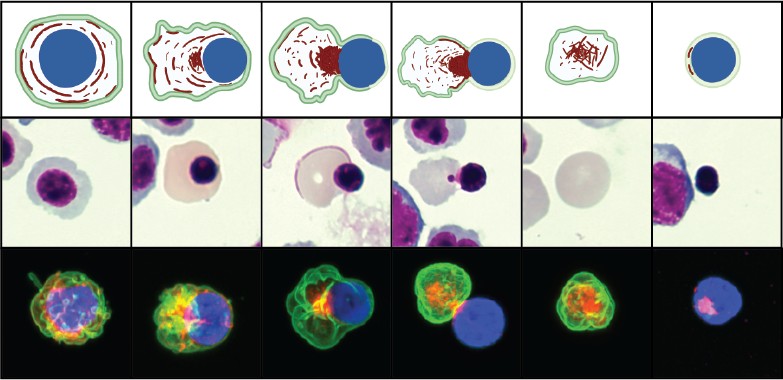
We study erythroblast enucleation in hematopoietic organs (bone marrow, spleen, and fetal liver) from transgenic mice and in human hematopoietic stem cells with actin cytoskeleton mutations. Biogenesis of mammalian RBCs (erythropoiesis) is a highly orchestrated process of terminal differentiation in which regulated gene expression directs a series of cell divisions coupled to dramatic changes in cell and nuclear morphology, culminating in cell cycle exit and nuclear expulsion (enucleation). While enucleation has been described morphologically for decades, the molecular mechanisms that drive the process remain unclear. We study erythroblast enucleation in hematopoietic organs (bone marrow, spleen, and fetal liver) from transgenic mice and in human hematopoietic stem cells with actin cytoskeleton mutations. We identify critical components by genetics, proteomics and biochemistry, analyze 3D cell shape and cytoskeleton architecture using confocal and super-resolution fluorescence microscopy, observe dynamics of fluorescent-tagged cellular components using time-lapse microscopy of living erythroblasts, and determine enucleation forces using computational modeling. These studies will elucidate molecular mechanisms of human congenital anemias due to defects in erythropoiesis and enucleation and provide strategies for optimizing RBC production in vitro for future applications in transfusion medicine.
Read more about the actin cytoskeleton and enucleation.
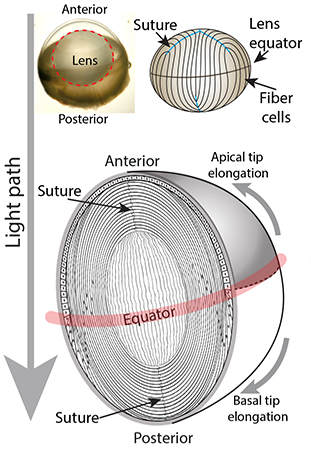
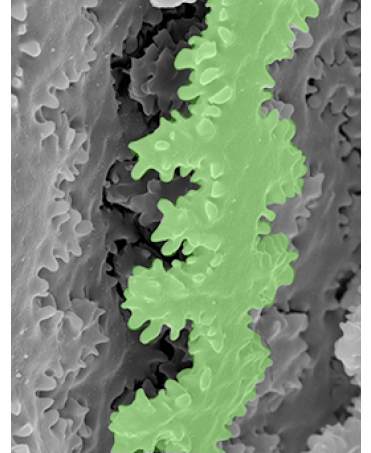
The lens functions to fine focus light onto the retina to produce a clear image. For proper functioning, the lens not only has to be clear but also must be an appropriate size, shape, and mechanical stiffness. Age-associated conditions of the lens include cataracts (lens cloudiness) and presbyopia (difficulty to focus on close objects). Despite decades of study, the exact mechanisms underpinning these aging conditions remain unclear. Using transgenic mice and other mammalian model systems combined with genomic, proteomic, and biochemical approaches, we aim to identify the actin cytoskeleton components that determine lens growth, cell sizes and shapes, and their microstructural mechanical responses. We utilize electron microscopy and high-and super-resolution fluorescence confocal microscopy to evaluate the complex 3-dimensional shapes and structures of lens cells. Additionally, by utilizing specialized equipment we mechanically compress or stretch lenses to evaluate biomechanical properties, and collaborate with engineers and computational biologists to model forces within the lens. This will enable us to understand how the actin cytoskeleton establishes lens size, shape, and biomechanical properties, and how cytoskeleton dysfunction may contribute to cataracts and/or presbyopia.
Read more about lens fiber cell hexagonal packing.
Listen to an interview about age-related changes in ocular lens biomechanics.